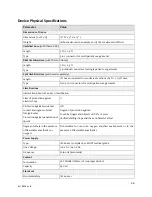
28
IFU-001 Rev. 8
16
C
ONTRAINDICATIONS
Pneumatic Compression Therapy
Do not use the device without a licensed healthcare practitioner prescription
.
NOTE: The prescription must show the time, temperature range, pressure, duration, and frequency of use of the
device. Make sure you fully understand the use of the device before starting
.
The patient should NOT use the therapy system if the patient is suspected of or observed to have any of the
following pre-existing conditions.
•
Presumptive evidence of
congestive heart failure
•
Pre-existing DVT condition
•
Deep acute venal
thrombosis
(Phlebothrombosis)
•
Inflammatory phlebitis
process
•
Episodes of pulmonary
embolism
•
Pulmonary edema
•
Acute inflammation of the
veins (Thrombophlebitis)
•
Decompensated cardiac
insufficiency
•
Arterial dysregulation
•
Erysipelas
•
Carcinoma and carcinoma
metastasis in the affected
extremity
•
Decompensated hypertonia
•
Acute inflammatory skin
diseases or infection
•
Venous or arterial occlusive
disease
•
Venous or lymphatic return
is undesirable
•
Poor peripheral circulation
•
Severe arteriosclerosis, or
active infection
Contraindications for Heat and Cold Therapy
Do not use the device without a licensed healthcare practitioner prescription.
NOTE: The prescription must show the time, temperature range, pressure, duration, and frequency of use of the
device. Make sure you fully understand the use of the device before starting.
The patient should NOT use the therapy system if the patient is suspected of or observed to have any of the
following pre-existing conditions.
Do not use on patients with Raynaud’s phenomenon or other vasospastic conditions, cold allergy, cold agglutinin
disorders like paroxysmal cold hemoglobinuria, Buerger’s disease, Chilblains, cryoglobulinemia, sickle cell
anemia, diabetes, hypersensitivity to cold or heat, history of cold injury, severe cardiovascular disease, anesthetic
skin, hypercoagulation disorders, poor circulation, extremities sensitive to pain, extremely low blood pressure
that are incapacitated, decreased skin sensitivity, vein ligation or recent skin grafts, or pheochromocytoma.
While using the device you should check the skin condition every hour for increased redness, discoloration,
itching, swelling, blisters, irritation and other changes. If any unusual conditions occur, immediately discontinue
using Therm-X and contact your physician.
Exercise special precautions for children under 12, pregnant users, hypercoagulation disorders, diabetes,
neuropathies, arthritic conditions, diabetes peripheral vascular disease, and patients with decreased skin
sensitivity.
Check for moisture on the therapy garment before placing on the skin. Remove any moisture before use.
The following patients must use Therm-X for temperature therapy under the supervision of a physician if they
are:
o
Patients with extremities not sensitive to pain
o
Patients with Extremely low blood pressure
o
Patients with Rayna
ud’s disease
o
Hypersensitivity to cold
o
Children under 12
o
Diabetics
o
Incapacitated patients
o
Patients with decreased skin sensitivity
o
Patients with poor circulation
o
Patients with vein ligation or recent skin grafts
Summary of Contents for Therm-X Series
Page 1: ...1 IFU 001 Rev 8...
Page 2: ...1 IFU 001 Rev 8...
Page 3: ...2 IFU 001 Rev 8 Welcome to the Team We re glad you re with us...
Page 4: ...3 IFU 001 Rev 8 QUICK START...
Page 21: ...20 IFU 001 Rev 8...