Oxygen Monitor SGM7.2.4/7.2.6 6 Composition
17
6.1.2
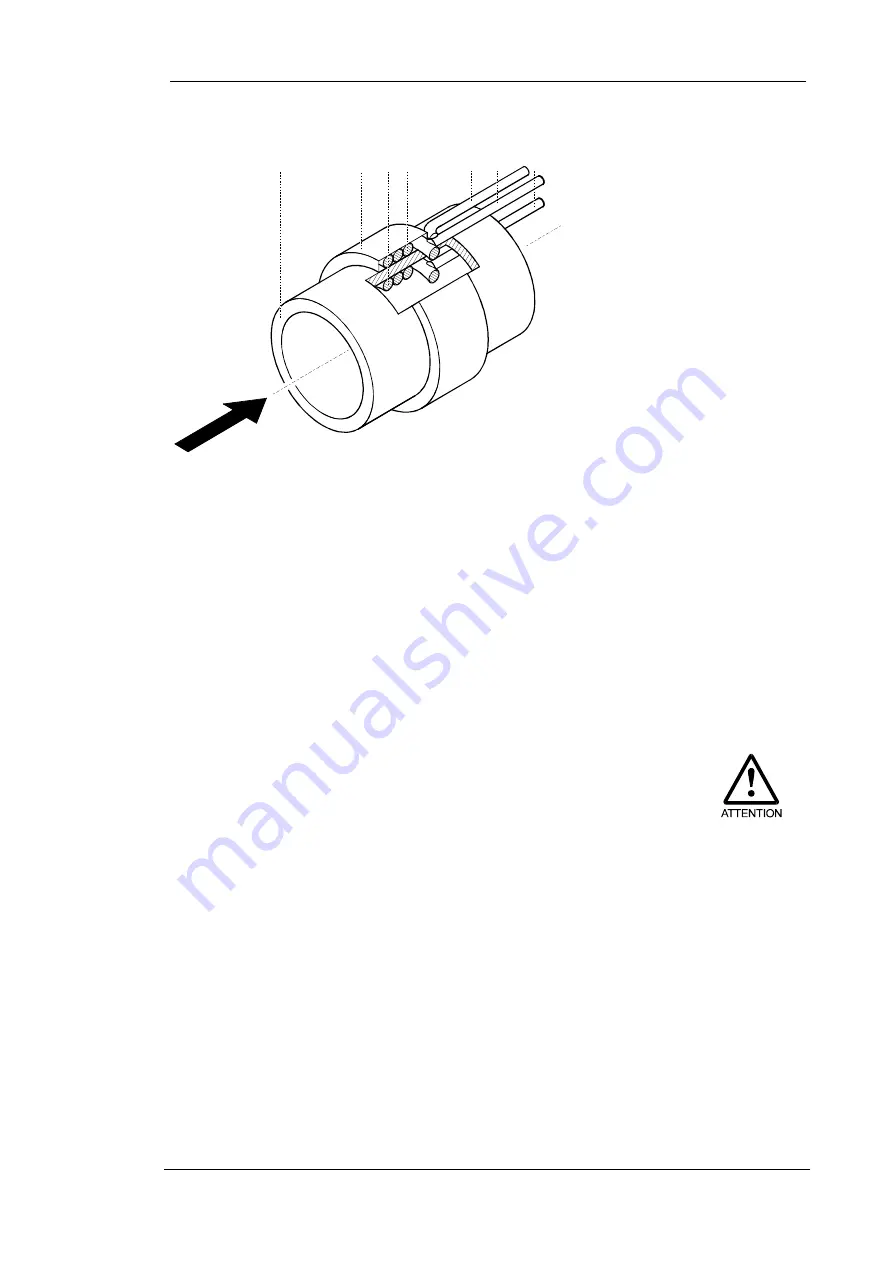
Construction principle of the solid electrolyte sensor
1
2
5
6
7
3
4
Measurig Gas
Fig. 2:
Composition of the solid electrolyte sensor
The measuring cell (sensor) consists of a tube made of zirconium
dioxide (2/1) with two platinum electrodes. The measuring electrode
is inside the tube (2/3). The reference electrode (2/4) is located on
the outside of the tube and has a constant electrode potential. The
electrodes and the ceramic tube form a galvanic solid electrolyte
measuring cell.
Measuring cell
(sensor)
In order to gain a higher oxide ion conductivity of the zirconium
dioxide tube, the sensor is heated to 750°C. This also avoids
interfering reactions with combustible components of the measuring
gas at the electrode caused by chemical unbalances. A thermocouple
(2/5) inside the measuring cell determines the actual electrode
temperature. A regulator ensures a constant temperature.
Sensor heater
The heated measuring cell produces thermal energy. Therefore, the
SGM7.2.4 must not be covered.
1 Ceramic tube
2 Ceramic cover of reference
electrode
3 Measuring electrode
4 Reference electrode
5 Thermocouple
6 Connecting wire of
reference electrode
7 Connecting wire of
measuring electrode
















































